Draw the number of electrons in the 1st, 2nd, and 3rd orbitals around the nucleus of sodium and chlorine.
Protons equal the Atomic Number. Neutrons are the Atomic Mass - Atomic Number. Remember the first orbital has 2 electrons maximum, the 2nd has 8 electrons, and the 3rd has 8 maximum.
How can the sodium and the chlorine "bond" to create a stable molecule of sodium chloride (halite)?
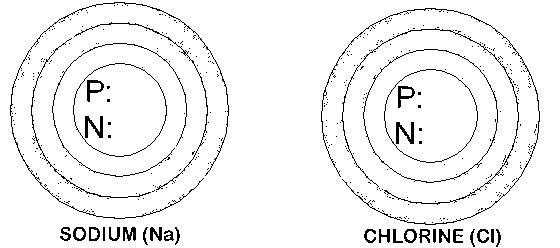
| Name: Sodium Symbol: Na Atomic Number: 11 Atomic Mass: 23 |
Name: Chlorine Symbol: Cl Atomic Number: 17 Atomic Mass: 35 |