BACKGROUND:
 The study of minerals requires a knowledge of atoms,
molecules, elements, and compounds. Rocks are made of minerals. Minerals are
made of elements. Elements are made of molecules, and molecules are made of
atoms.
The study of minerals requires a knowledge of atoms,
molecules, elements, and compounds. Rocks are made of minerals. Minerals are
made of elements. Elements are made of molecules, and molecules are made of
atoms.
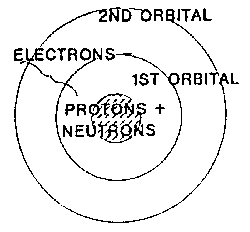
Elements are composed of extremely small particles
called atoms. An atom is the smallest part of an element that retains all
the characteristics of the element. Atoms are composed of protons, neutrons,
and electrons, which is composed of the basic building blocks of mater,
leptons and quarks. . These subatomic particles lack the distinct
characteristics of elements.
Protons and neutrons are made of
different combinations of quarks. There are six different types of
quarks known as flavors: up, down, charm, strange, top, and bottom.
Protons are positively charged, and neutrons are electrically neutral.
Neutrons and protons are found within the nucleus of an atom.
Electrons are one of the six types of
leptons. Electrons in elements are found a space from the
nucleus at distinct
distances called atomic orbitals. Each of the orbitals can contain a set number of
electrons, but it is difficult in this model to describe where the electrons
can be found. Hydrogen has one electron which represents
the lowest energy level. Neon with atomic number of 10 has an
atomic configuration of 1s2 2s2 2p6.
Neon is one of the noble gases because its outer orbitals have enough
electrons to be full. However, some elements like Magnesium
have a Neon like orbital structure, except they have 2 electrons in a third
shell ( 1s2 2s2 2p6 3s2).
Magnesium's third shell is not full.
Most substances found on the Earth usually consist of
more than one atom. When two or more atoms form a chemical bond, they create
a molecule. A molecule can consist of two or more atoms of the same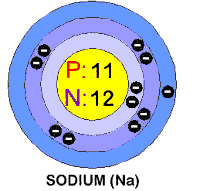 element
(O2) or of atoms of two or more different elements (H2O).
When two or more elements combine, they form what is called a compound. A
molecule is the smallest distinct part of an element or compound.
element
(O2) or of atoms of two or more different elements (H2O).
When two or more elements combine, they form what is called a compound. A
molecule is the smallest distinct part of an element or compound.
In this lab, students will consider halite (table salt)
which has the chemical formula NaCl. Sodium (Na) and chlorine (Cl) are very
different elements.
In its elemental state, chlorine is a
greenish-yellow gas that has a melting point of -101 degrees centigrade and
a boiling point of -34.1 degrees c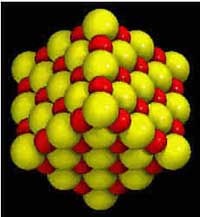 entigrade. It has 17 electrons in three
shells. It also has an atomic diameter of 3.62 units (don't worry about
the type of units). Sodium melts at 98 degrees centigrade and boils at 889
degrees centigrade. Elemental sodium is a silvery-white metal that can be
cut by a knife. It reacts very violently with air or water. It is an
excellent conductor of heat and electricity. It has 11 electrons, also in
three shells. The atomic diameter of sodium is 1.81 units (½ the size of
a chlorine atom).
entigrade. It has 17 electrons in three
shells. It also has an atomic diameter of 3.62 units (don't worry about
the type of units). Sodium melts at 98 degrees centigrade and boils at 889
degrees centigrade. Elemental sodium is a silvery-white metal that can be
cut by a knife. It reacts very violently with air or water. It is an
excellent conductor of heat and electricity. It has 11 electrons, also in
three shells. The atomic diameter of sodium is 1.81 units (½ the size of
a chlorine atom).
Although sodium and chlorine are very different, they
combine to form the compound called halite (NaCl), commonly known as table
salt. Halite crystals grow in a cubic form, which reflects how chlorine and
sodium combine. Since a sodium atom is half the size of an atom of chlorine,
the two elements combine perfectly in a cubic pattern, as shown to the left.
PROCEDURE:
 Review the structure of atoms with your students. Hand out the
periodic table placemats. Review the common elements and their symbols.
Call out the name of an element, and ask the students give you the
symbol. You may want to ask other questions, such as the atomic weight
or whether the element is normally a solid, liquid, or gas.
Review the structure of atoms with your students. Hand out the
periodic table placemats. Review the common elements and their symbols.
Call out the name of an element, and ask the students give you the
symbol. You may want to ask other questions, such as the atomic weight
or whether the element is normally a solid, liquid, or gas.
- Ask the students to locate sodium (Na) and chlorine (Cl) on the
placemats. Discuss them in detail as outlined above. Have the students
fill in the worksheet, using the information from the periodic chart.
Review electrons, protons, and neutrons.
- Give students a small amount of salt to examine. Ask them if the salt
is cubic. (the answer is yes). Challenge the students to find a crystal
that is not cubic. (There may be clumps of cubes, but these are still
cubic.) Ask them if these are crystals, or crystals that have been
broken into little cubes. (The answer may vary, but when broken or
"cleaved," salt will still form cubes.)