BACKGROUND:
An atom consists of three basic components: electrons,
protons, and neutrons. An electron has a negative charge; a proton has a
positive charge; a neutron has no charge. An atom that has a charge is
called an "ion." If an ion has too many electrons, it has a
negative charge. If an ion has too many protons ( not enough electrons) it
has a positive charge. Positive and negative ions will combine to form
neutral substances.
When a solute is dissolved in a solvent, the compound
is "broken" into its consistent atoms, which are almost always
ions. These ions will easily be attracted to other ions if given the chance.
Positively charged ions will be attracted to negatively charge ions.
In this lab, the students will experiment with the
bonding behavior of elements through electroplating (the art of producing
metallic coatings by using electric currents.) Economically, metallic
coatings are used to improve appearance, resist corrosion, or improve
hardness. Examples include plating steel with copper, nickel, or chromium in
the automotive industry; tin plated steel for food cans; and the manufacture
of silver or gold plated jewelry.
In this lab, the students will make a solution of
copper sulfate in water. When water is added to the copper sulfate, the
copper sulfate is broken into copper ions (Cu2+) and sulfate ions
(SO42-). The copper ions roam around in the solution
looking for a negatively charged with which to combine.
This process is facilitated by passing an electric
current through the solution. In this lab, the power source is a battery. As
directed below, a copper strip is wired to the battery’s positive terminal
(the anode), while a zinc or iron strip is attached to the negative
terminal (the cathode). When electricity flows through the circuit, the
positive copper ions in solution bond to the negatively charged metal. In
addition, the copper strip loses Cu2+ ions, which replace the Cu2+
ions lost from the solution. Given time to experiment, any substance can
eventually be electroplated. Students will see that elements can
"move" around with just a small amount of energy from the battery.
PROCEDURE:
- Reinforce to students that chemical compounds are made of elements,
which are composed of atoms. This lab will demonstrate how elements by
becoming ions can be manipulated and moved from one area and deposited
in another. Emphasize that it is not magic when the elements move
around, but their response to the electrical current.
- Perform the electroplating experiment, following the directions below.
- Mix the solution. Use 250, 500, or 1000 ml glass beakers. For
every 250 ml of water, use approximately 5 ml of copper sulfate.
Copper sulfate is included in the kit.
Note: After the experiment, you can safely dispose of the
solution down the sink. However, the solution is reusable for a few
years. If you keep it, store in it safe place, correctly marked as
copper sulfate solution.
- Place the electroplating materials on a table. Make sure that the
beakers and electrodes are clean. . Use alligator
clips from the Electricity Kit to attach the battery to the copper
and zinc strips that are included in the Rock Cycle - Chemistry (6)
kit.
-
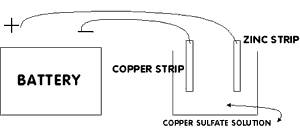 Using
the alligator clip, connect the wire wrapped to the piece of copper
to the POSITIVE battery terminal (see the diagram). Connect the
other wire to the NEGATIVE battery terminal. You should now have one
wire from the positive terminal connected to the copper electrode
and one wire from the negative terminal connected to the iron or
zinc electrode.
Using
the alligator clip, connect the wire wrapped to the piece of copper
to the POSITIVE battery terminal (see the diagram). Connect the
other wire to the NEGATIVE battery terminal. You should now have one
wire from the positive terminal connected to the copper electrode
and one wire from the negative terminal connected to the iron or
zinc electrode.
- Place the electrodes in the solution and let the experiment stand.
You should begin to see little bubbles form on the copper electrode
very soon. If you wire the electrodes backwards, a different
reaction will occur; a black film will coat the electrode. If this
happens, clean all the metal by wiping it with a tissue, and try the
experiment again.
- Let the electrodes stay in solution for about 5 minutes. When
removed, the anode (positive) should be coated with copper.
You can replace the zinc strip with other metals and see what
happens (i.e. nickel).