Let’s look at some examples.
Hydrogen has one electron
which represents the lowest energy level. Neon with atomic
number of 10 has an electron configuration of 1s2 2s2
2p6. Neon is one of the noble gases because its outer
orbitals have enough electrons to be full. However, some elements like
Magnesium have a Neon-like orbital structure, except they have 2
electrons in a third shell ( 1s2 2s2 2p6
3s2) . Magnesium's third shell is not full. In the chapter
on “Atomic Theory” we will look at these models in more detail and how
they historically developed into workable models of today. However,
with added information, even these models may change to fit new data.
|
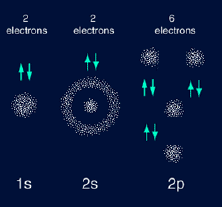
Neon
|
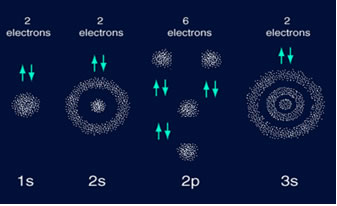
Magnesium
|
|
|