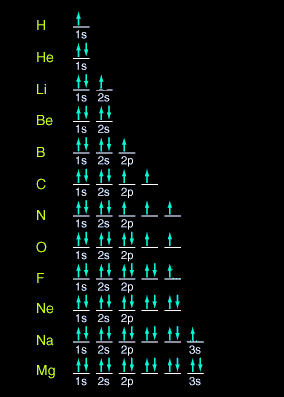
Valence shell configuration representation |
Elements are composed of extremely
small particles called atoms. An atom is the smallest part of an
element that retains all the characteristics of the element. Atoms are
composed of protons, neutrons, and electrons, which
is composed of the basic building blocks of matter, leptons and
quarks. These subatomic particles lack the distinct
characteristics of elements.
Protons
and neutrons are made of different combinations of quarks.
There are six different types of quarks known as flavors
including up, down, charm, strange, top, and bottom.
Protons are positively charged, and neutrons are electrically
neutral. Neutrons and protons are found within the nucleus of an
atom.
Negatively charged
particles called electrons and are one of the six types of
leptons. Electrons are found away from the nucleus at distinct
distances from the nucleus called atomic orbitals or energy
levels. Each of the orbitals can contain a set number of electrons,
but it is difficult in this model to describe exactly where the
electrons can be found.
On the Periodic
Table of the Elements you can determine orbitals looking at the “Valence
shell configuration.” This configuration is an advanced look into
an elements atomic structure. On the recommended Periodic Table, you
can find this number on the lower right hand corner. Atomic orbitals
are mathematically derived and describe the motion and placement of
electrons of an atom. Early researchers thought that electrons used to
orbit the nucleus similar to planets revolving around the Sun. However,
an electron is not solid like a planet, so the term orbital is
now used instead of orbit. The orbitals are named s (sharp),
p (principal), d (diffuse) and f (fundamental). The
usage of s,p,d, and f were used because of their relationship to
spectral characteristics to determine the number of electrons.
However, spectral analysis is no longer used but chemists still keep the
letters.
|
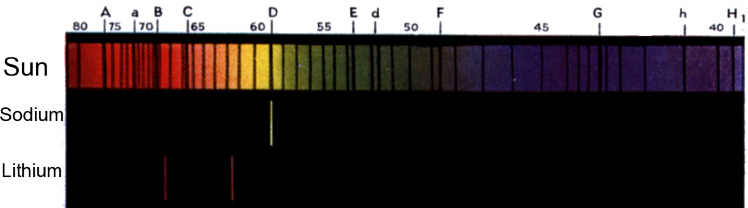
Spectral characteristics of sodium and lithium compared to solar
radiation. |
|