|
Electroplating a key with copper |
Electrolysis
of pure or distilled water is very slow. You can speed it
up by adding an electrolyte such as an acid or base.
Salts like epsom salt, works very well.
The
overall reaction is 2H2O(liquid)
→ 2H2(gas)
+
O2(gas)
Electrolysis
is important in other applications especially in the manufacture of
sodium, hydrogen, oxygen, fluorine, aluminum, and chlorine.
Electroplating which is used to put a thin layer of certain metals
on other metals is a direct application from the invention of
electrolysis.
Now, let’s make some hydrogen and
oxygen from water.
|
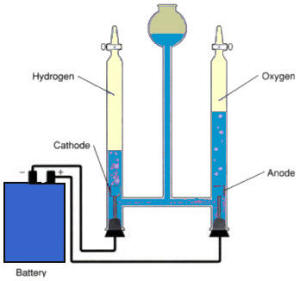
Electrolysis set-up |
Connect one end of each of the two
alligator clips to a carbon rod. The carbon rods are easily
damaged and broken so care must be used. Connect the remaining ends of
the two wires to the connectors on the 6 volt battery. Add 200 ml of
water to a 250 ml beaker. To this solution add about 1 tablespoon of
Epsom salt. Fill each of the two test tubes or 10 ml graduated cylinders
with some of the water and Epsom salt solution. Immerse the carbon rods
into the graduated cylinders. Quickly flip the graduated cylinders over
and place them in the beaker. The majority of the carbon rod should be
inside the graduated cylinder. There should also be enough water in the
graduated cylinder so that the carbon rods are completely immersed.
Once the graduated cylinders are both
placed in the beaker, bubbles will start to form on the carbon rods. The
formation of the bubbles indicates the generation of hydrogen and oxygen
gas. Because water is composed of 2 parts hydrogen to one part oxygen,
the carbon rod creating hydrogen gas will show twice as much activity as
the rod evolving oxygen. This can be verified using the graduated
cylinder (provided that not much water has been lost during initial
transfer into the beaker.) The graduated cylinders will capture the
gases and the displacement of water can be measured using the graduated
cylinder.
To stop the
experiment, simply disconnect the battery. The presence of hydrogen gas
can be verified using a lit wooden stick. Since hydrogen is explosive,
exposing a small volume to a flame will result in a "pop." Be
careful and listen to all instructions. To check for oxygen use a
glowing wooden splint (flame has been blown out) and place it in the
test tube with oxygen. The splint will flame up in oxygen |