|
Ionic bonds

Irving
Langmuir |
|

Richard Abegg

Gilbert Lewis |
Richard
Abegg (1869-1910), a German chemist concluded that the Noble gases
(i.e., Argon) were stable because there was 8 electrons in the outermost
shell. If that is true, then maybe the electrons of other elements
exchanged electrons to create a stable molecule. He proposed the
“valence bond theory,” which began to explain how atoms bond with
each other. This led the way to understanding the principle of ionic
bonding. Abegg was killed in a balloon accident, so his theory had to
wait for other scientists to take on the cause.
In the early 1900’s German chemist
Walther Kossel (1888-1956) and the American chemists Irving
Langmuir (1881-1957) and Gilbert Newton Lewis (1875-1946)
independently discovered the ionic chemical bond. They developed
another way in which bonds can occur by sharing electrons in their other
shells
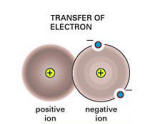
Basically, ionic bonding is when an
atom does not have the full number of electrons in each orbital;
it seeks a partner that can "loan" one or more electrons to "fill" its
molecular orbital. This is the essential cause of chemical bonding.
Let’s revisit halite (NaCl), a sodium ion(+), which has a
positive charge wants to give up an electron whereas a chlorine
ion(-), which has a negative charge wants to accept an
electron. The two elements combine to form a bond by the attraction of
unlike charges, thus forming the compound, NaCl.
|