|
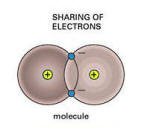
Covalent bond

Kekule |
|

A. S. Couper |
Understanding the
electron was a key discovery in understanding how elements
interact or chemically bond with each other. It was puzzling to
have neutral particles to come together as proposed by earlier atomic
theories. The discovery of electrons by J.J. Thomson provided
clues about how bonding occurred.
In 1852,
E. Frankland (1825-1899), an English chemist first proposed the
concept of a valence. Different elements had specific number of
electrons in their outer or valence shell. German chemist
August Kekule (1829-1896) was first to propose the term “covalent
bond” when describing the large molecule that formed due to
multiple chemical bonds of carbon. Scottish chemist Archibald Scott
Couper (1831-1892) independently discovered self-linking carbons.
They proposed that carbon had a valence of four and shared electrons.
This was the first description of covalent bonding.
Couper
also proposed a way to write the coupling on paper by using a straight
line linking symbol for atoms which is still in practice today. For
example, a molecule of water could be represented by the structural
formula: H-O-H. The arrangement of atoms in a molecule is
represented by the symbols of the elements present joined by dashed
lines that show how the atoms of those elements are bonded to each
other. This is referred to as the Couper dash system.
|