|

Erwin Schrödinger |
Austrian physicist Erwin Schrödinger (1887-1961) developed
an “Electron Cloud Model” in 1926. It consisted of a dense
nucleus surrounded by a cloud of electrons at various levels in
orbitals. Schrödinger and Werner Heisenburg
(1901-1976) mathematically determined regions in which electrons
would be most likely found. The probability of the finding the
electrons in the orbitals are sometimes referred to as “lobes.”
They used the mathematical equations for the behavior of waves
following the work on waves by Louis de Broglie (1892-1987)
a French theorist.
|
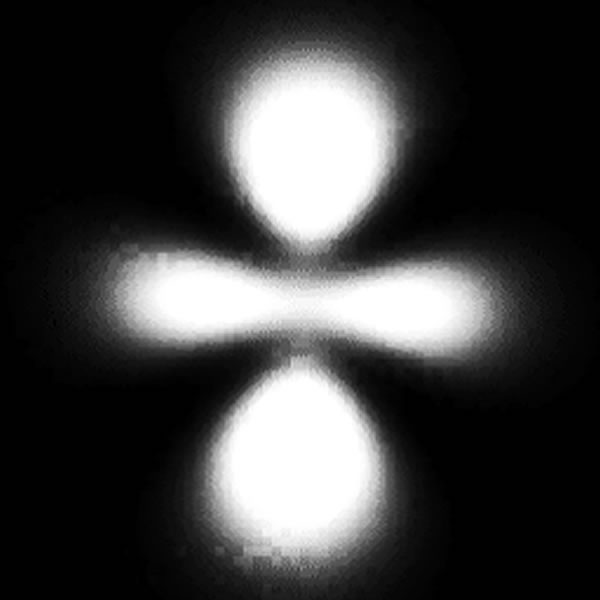
Electron "cloud" |
It is still impossible to see a
single atom, even with the world’s best microscopes, but we can
see images of groups of atoms, and the trails that they leave.
Starting in the 1950’s, experiments using the newly invented
particle accelerators and particle detectors opened up a new age
of “particle physics.” Through the last half century
individual particles were identified by teams of researchers in
only certain facilities around the world. Fermilab in
Illinois, Stanford Linear Accelerator Center (SLAC) in
California, Brookhaven in New York, CERN the
European Laboratory near Geneva, Switzerland, and DESY
in Hamburg ,Germany will continue to refine the individual
particles with every experiment. They are still working on
discovering particles that will fully prove a Standard Model,
which not only explains how atoms work, but how atoms are part of
a Unifying Theory. We will visit this again in later
chapters.
|