|
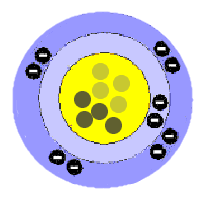
Bohr Planetary Model

James Chadwick
|
Danish physicist Niels Bohr
(1885–1962) in 1913 created a model that allowed electrons to travel
without losing energy by going in defined orbits. He related it to
the orbits of planets around the Sun. This model fit much of the
data at that time; a nucleus with protons and electrons revolving around
the nucleus. The number of protons equaled the number of
electrons. However, there was a problem that the total mass of an
atom was greater than the sum of the protons and electrons.
|

Niels Bohr on left with friend Albert Einstein. |
English physicist Sir James
Chadwick (1891–1974) in 1932 solved this problem by introducing the
neutron. He deduced that a neutron is a particle with no
electric charge or is “neutral.” So Chadwick refined the Bohr model to
include neutrons within the nucleus. This model proved well for
chemists, but physicists were still having problems as other theories of
relativity, quantum theory, and uncertainty were
challenging the Bohr model.
Bohr continued to refine the model as
he realized that electrons can be excited to jump up or down an orbital
releasing energy. The term orbital was now used so there would not be
confusion with orbits of the planets. Bohr used these observations to
theorize that energy of an electron is “quantized,” or that certain
quantities of energy can be created.
Mathematical models
help to predict charge, mass, speed, spin, and other properties of
electrons. The Bohr Model no longer could satisfy the results. It
provided enough evidence, that a new model was needed. |