BACKGROUND:
 Water
is very important to our everyday
lives because of the manner in which hydrogen and oxygen "hold" hands.
The water molecule is very strong, but the way it is arranged, allows many
other substances that can "hide" between the hydrogen and oxygen.
What makes water so important? Water is a peculiar substance with
properties that make it an ideal fluid. If you theoretically calculate
the boiling and freezing temperatures of water you will find that water
has an unusually low freezing point and high boiling point compared to
other molecules that have similar structures (sulfur, selenium, and
tellurium).
Water
is very important to our everyday
lives because of the manner in which hydrogen and oxygen "hold" hands.
The water molecule is very strong, but the way it is arranged, allows many
other substances that can "hide" between the hydrogen and oxygen.
What makes water so important? Water is a peculiar substance with
properties that make it an ideal fluid. If you theoretically calculate
the boiling and freezing temperatures of water you will find that water
has an unusually low freezing point and high boiling point compared to
other molecules that have similar structures (sulfur, selenium, and
tellurium).
How can you explain such a big difference?
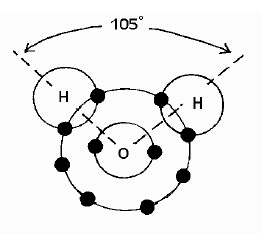
The molecular structure of water resembles that of Mickey Mouse's or a teddy
bear's head
(figure above). The hydrogen and the oxygen have a very tight covalent
bond, where the hydrogen and the oxygen share electrons as they dance and
twirl around in the molecule. The individual molecules of water are
also held together very tightly by what is specifically called
a hydrogen bond. A hydrogen bond is much stronger than other bonds
that molecules have. Ionic bond is one of those weaker bonds, and
substances like salt can be easily be broken up. Water is a package
of power that is hard to break, and it is this strength that allows other
substances to dissolve or break up in water, hence the name, universal
solvent.
PROCEDURE:
- If students are not familiar
with using a protractor go over how to determine angles with the paper protractor. You may want them to cut them out and show them how to
measure.
- Use the circle on the worksheet to figure out the
different angles depending on how much practice they may need.
- Instruct the students to use two of the hydrogen and one oxygen to make
a representative model of how the elements are bonded.
The two hydrogen’s are 105º between each other as in the diagram in
the background information.