BACKGROUND:
It is difficult to distinguish between the hardness of
a mineral and the ease with which a mineral may be broken. Hardness refers
to the ability to scratch the mineralís surface. However, some hard
minerals, like diamond and quartz, break easily if dropped. Hence mineral
breakage is different from hardness. Minerals break in two ways: fracture
and cleavage. Fracture is irregular breakage. Cleavage is a regular breakage
that follows the atomic structure of a mineral. Cleavage results in smooth,
planar surfaces. Different minerals may have one, two, three, four, or six
cleavages.
Mohs hardness scale is used by geologists to compare
the hardness of minerals only. The scale arranges a series of
minerals in order of increasing relative hardness, from 1 to 10. Note that
this is a relative hardness scale; diamond is actually over four hundred
times harder than talc.
PROCEDURE:
- Draw the Mohs hardness scale on the board. Ask the
students which of their lab samples are part of the scale. Ask them if
they think the scale is useful. Tell them that the scale works well in a
laboratory, but in the field, a geologist would not have all 10 minerals
available. Geologists usually use their fingernails and steel knives.
- Explain that the Mohs scale does not explain why
some minerals are harder than others. Ask students to draw a large
person that weighs 250 lbs. and a muscular person that weighs 250 lbs.
Ask them if one person is "softer" than the other. One person
works out more, and the cells of that body combine tightly, giving him
or her a different appearance. The elements of some minerals do the
same. The ones that are tightly bound together look different than do
ones with looser bonds.
For example, in the illustrations below, (A) shows
the atomic structure of carbon in a diamond, and (B) is the carbon
arrangement in graphite. (A) is more compact than (B), hence it is
harder. As an example, you can tell the students that when Superman
squeezes a piece of carbon in his hand, it turns into a diamond.
(Superman usually uses coal, which is not the right source of carbon,
since the substance should be inorganic to be a real mineral.) If
desired, have the students construct Googolplex models of graphite
and diamond. Use the directions provided with the Googoplex models. You
can also use the Zometool system to construct similar models.
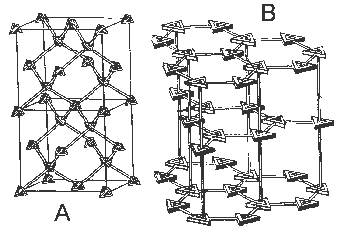
Examples of crystalline structures
|