BACKGROUND:
The atoms in crystalline solid matter are arranged in
regular, repeating .patterns. All other types of solid matter are amorphous
or without a regular atomic arrangement. Metals and minerals are
crystalline. Glass is amorphous. Depending upon its composition, the
crystalline pattern of a mineral may not be visible in a hand sample. In
this case minerals are studied using X-ray diffraction, a technique that
uses the reflection of X-rays to determine crystal structure and
composition.
PROCEDURE:
-
Draw the following diagram on the board to
illustrate crystalline versus a non-crystalline (amorphous) patterns.
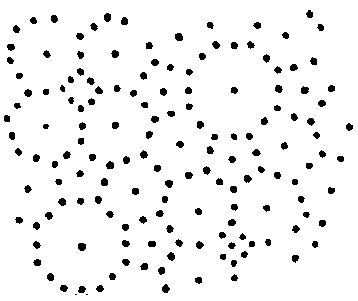
crystalline
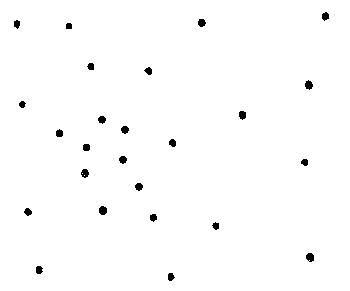
amorphous
-
On the worksheet, have the students
outline or fill in spaces on the Altair designs sheet to create patterns.
Their patterns are examples of order within the overall structure of the
design. This same type of organization generates crystalline structures in
minerals. The Altair designs sheet will naturally guide each studentís
imagination through the maze of lines. Since no two students are alike,
none of them will see the same shapes, forms or patterns hidden in these
designs. They may create some very interesting artwork.
-
After the students finish their
patterns, see if there are any similar patterns. Use any similarities and
differences to reinforce that there are many types of minerals, and hence
many different crystal patterns.
|