BACKGROUND:
All known substances can be classified as solids,
liquids, gases, or plasma. In addition, a fifth state of matter, the Bose-Einstein
condensate has been discovered recently. However, it is not stable at normal
earth conditions. Likewise, although plasma is the most abundant state of
matter in the Universe, it is not common on the Earth under normal
conditions, except for lightning. Most matter that students are familiar
with will therefore be in a solid, liquid, or gaseous state.
An element is a pure substance that cannot be
decomposed into simpler substances by normal chemical means. There are 109
different elements. Ninety of these are naturally occurring; the rest have
been created in laboratories. Elements 110 and 118 are still being
researched on. There will be more elements as technology can identify them.
A symbol is used to represent the full name of an element. For example, H
represents hydrogen; O represents oxygen, and Al represents aluminum.
Sometimes the Latin name for an element is used as the basis for its symbol,
for instance K represents potassium (kalium in Latin).
Three subatomic particles compose elements: protons,
neutrons, and electrons. Protons, which have an electrical charge of +1, and
neutrons, which have a neutral charge, make up the nucleus of an element.
This nucleus is surrounded by a "cloud" of electrons, each of
which as a charge of -1. The electrons spin around the nucleus in what are
called orbits or shells. Each of the orbits can contain a set number of
electrons. For instance, the first orbital from the nucleus has 2 electrons,
the second has 8, the third has 8, the 4th has 16 and the fifth has 32, and
so on. Each shell may not be full, depending on the number of electrons in
the element, and the inner shells fill before the outer shells fill. Sodium,
for example, has 11 electrons, which are located in the first, second, and
third shells (2+8+1.)
An element has a uniform composition. Different
elements may join together; these combinations are called compounds. A
compound can be separated into its component elements by chemical means. For
example, common table salt is a compound made of two elements: sodium and
chlorine. Table salt can be broken down into sodium and chlorine by mixing
it with water. However, sodium and chlorine cannot be easily broken down
into any simpler forms.
PROCEDURE:
-
Discuss the properties of elements with the students. Review the
structure of the periodic table. Ask students questions about the
different elements and see if they can locate them on the periodic
table.
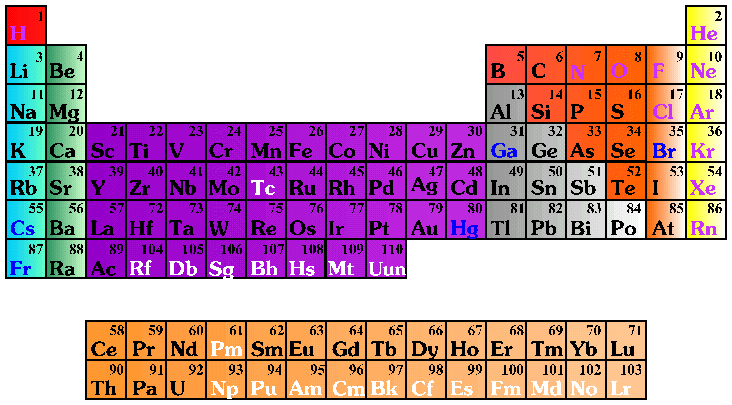
-
Review the difference between an element and a compound. The students
should realize that an element cannot be broken down, whereas a compound
can be subdivided into elements. You may wish to explain that in many
instances, forming or breaking down a compound requires energy. For
example, if you place a mixture of iron and sulfur in a bowl, they will
not react. No compound will form. However, if iron and sulfur are mixed
and then heated, they will combine and form a compound.
-
Write the following examples of compounds and their constituent
elements on the board. At this point, do not be concerned with
explaining the "endings" to the chemical words, such as
chlorine versus chloride. These endings reflect the molecular structure
of the compound.
|
ELEMENTS |
COMPOUNDS |
|
Na-sodium |
NaCl (sodium chloride) |
|
Cl-chlorine |
AgCl (silver chloride) |
|
K-potassium |
KCl (potassium chloride) |
|
Ag-silver |
KClO (potassium perchlorate) |
|
O-oxygen |
H2O (water) |
-
Use the Periodic Table placemats to explore elements with
the students. When they examine the chart, the students may ask the
meaning of the numbers surrounding the element symbols. The number in the
upper left corner is the atomic number, i.e., the number of protons
inside the nucleus of the element. The number in the lower left is the
atomic mass or atomic weight, which is essentially a measurement of how
heavy the element is.
-
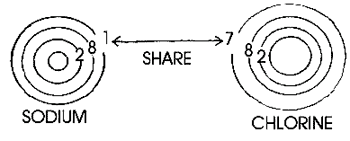 Explain
the basic subatomic structure of elements. Tell the students that protons
and neutrons reside inside the nucleus. The electrons spin around the
nucleus in what are called orbits or shells. Each of the orbits represents
a set number of electrons. For instance, the first orbit from the nucleus
has 2 electrons, the second has 8, the third has 8, the 4th has 16 and the
fifth has 32, and so on. Sodium for instance, has 11 electrons located in
the first, second, and third shells (2+8+1.) Explain
the basic subatomic structure of elements. Tell the students that protons
and neutrons reside inside the nucleus. The electrons spin around the
nucleus in what are called orbits or shells. Each of the orbits represents
a set number of electrons. For instance, the first orbit from the nucleus
has 2 electrons, the second has 8, the third has 8, the 4th has 16 and the
fifth has 32, and so on. Sodium for instance, has 11 electrons located in
the first, second, and third shells (2+8+1.)
|