|

John Newlands
|
John Newlands (1837-1898) an English chemist tried to classify elements by their
atomic weight, and noticed a repeating pattern of every eight elements.
He used the analog with music and called it the Law of Octaves.
However, determining atomic weights was based on comparing other
elements to the lightest element which was hydrogen and given a value of
1.00. Some of the elements were inaccurately given values. He was
ridiculed by other chemists who felt the table he created was not
reliable. He could not get his papers published and returned as chief
chemist in a sugar factory and later opened a chemical business with his
brother.
|

Mendeleev by Ilya Repin
|
Dmitri Mendeleev (1834-1907) rose from very poor beginnings to a position of a renowned
Russian chemist in the 19th century. He wrote down information on each
element on cards. He noticed that the atomic weight (we now
refer to as the atomic mass) could be used to rank the elements
from lightest to heaviest. Mendeleev noticed that if you looked at
their chemical properties there was a periodicity of properties,
especially if you left spaces where the properties did not fit. He
stressed that there was order to the elements, and that this order would
help predict elements not yet discovered. He used the calculated
atomic weights of the time (which had improved since Newlands) and used
them to put the elements in a table. Mendeleev predicted the properties
of aluminum, boron, and silicon. Gallium,
scandium, and germanium were also found to fit his
predictions. There were some discrepancies, but the format of the table
is the basis of today’s periodic table. Mendeleev also noted errors in
the atomic weight of some elements. Mendeleev’s table as published in
1869, had many gaps and questions. If you think it does not look like
today’s table, read the horizontal lines, and notice they are today’s
periodic table’s Groups I-VIII.
|
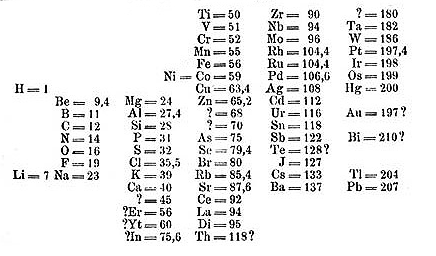
Table from Mendeleev's 1869 paper
|
|
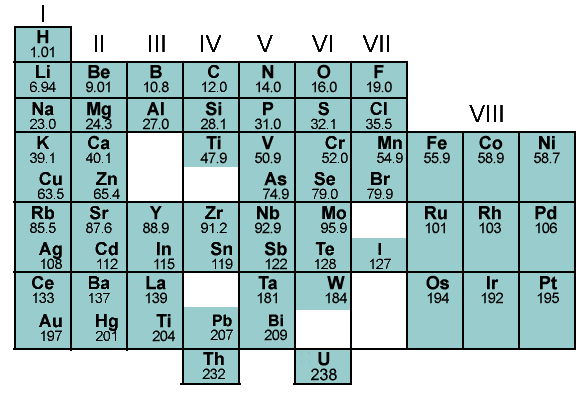
Mendeleev's elements (1869) compared to periodic "Groups." |
|