|
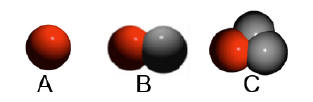
A=hydrogen; B=deuterium;
C=tritium |
The atomic
number refers to the number of protons per atomic nucleus.
Atomic mass (weight on many periodic tables) is the combined mass of
the protons and neutrons. You can subtract the atomic number from the
atomic mass and find the number of neutrons. The atomic mass on
most Periodic Tables includes the different isotopes of that
element, so the number is an average. An isotope is an element that has
the same protons, but its different isotopes
 have varying
number of neutrons. Hydrogen has three isotopes deuterium
with one neutron and tritium with two neutrons. If it has no
neutrons this is usually referred to as hydrogen. When a Periodic Table
gives you the atomic mass of all the isotopes, you should round off the
atomic mass to the nearest whole number to derive the number of
neutrons. The number of protons equals the number of electrons. have varying
number of neutrons. Hydrogen has three isotopes deuterium
with one neutron and tritium with two neutrons. If it has no
neutrons this is usually referred to as hydrogen. When a Periodic Table
gives you the atomic mass of all the isotopes, you should round off the
atomic mass to the nearest whole number to derive the number of
neutrons. The number of protons equals the number of electrons.
Each of the
elements has not only a unique atomic mass and number but also have a
crystal structure in which they form on the atomic level. The crystal
structure refers to the way in which the atoms come together to form
a pattern. On the recommended Periodic Table each of the natural
occurring elements will have a crystal symbol in the element box. Refer
to Symmetry of Matter, Lesson 5 for a further explanation (under Math
Skills).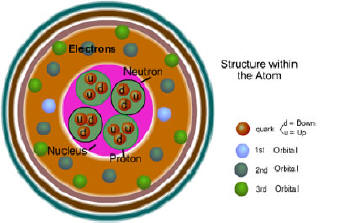
The importance of melting and
boiling points helps to define some physical properties of the
element. The melting point is the temperature that a substances changes
from a solid to liquid. The boiling point is when a material changes
from a liquid to gas. These properties help to measure the strength of
that element. Density is sometimes referred to as Specific
Gravity on some Periodic Tables. It is a physical property that
measures how a substance is “packed.”
|