  Carbon
atoms are closely associated with organic chemistry.
Biological carbon has the ability to form strong bonds with each
other and form large molecules. Hydrocarbons can build a network of
carbon atoms linked to hydrogen.
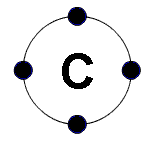
The carbon atom is tetravalent and
can bond to four other atoms. They can form endless closed rings or
continuous rows. There is no limit to the size and complexity of these
carbon molecules. Understanding hydrocarbons has allowed us to produce
products from gasoline, polyester, and plastics. Carbon
atoms are closely associated with organic chemistry.
Biological carbon has the ability to form strong bonds with each
other and form large molecules. Hydrocarbons can build a network of
carbon atoms linked to hydrogen.
The carbon atom is tetravalent and
can bond to four other atoms. They can form endless closed rings or
continuous rows. There is no limit to the size and complexity of these
carbon molecules. Understanding hydrocarbons has allowed us to produce
products from gasoline, polyester, and plastics.
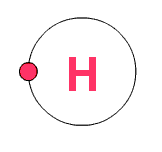
Hydrogen
has one electron in its outer orbit, but it would like to have two. That
means it tends to combine with other elements so that it will fill its
outer orbit with two electrons. Carbon has 2 electrons in the first
orbit and 4 electrons in second, outer orbit. It would need four more to
fill the orbit with the maximum of 8 electrons.
|

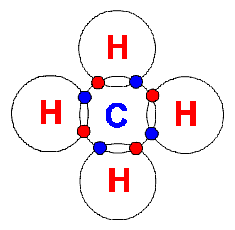
CH4 |
Hydrogen and carbon
atoms can combine to form hydrocarbon molecules with a single bond
(these are referred to as alkanes). Examples are Methane,
Ethane and Propane. Methane (CH4) is a
complete hydrocarbon and the main ingredient of natural gas. Each of
the hydrogen atoms have 2 electrons in their outer shell and carbon now
has a total of 6 (4 from the carbon atom, and 4 used from the 4 hydrogen
atoms). Ethane (C2H6) is a colorless,
odorless gas that is a by product of petroleum refining. It is a two
carbon alkane. It is important because it helps to make many other
hydrocarbons, especially ethylene (C2H4). Highly
complex hydrocarbon molecules are possible with these building blocks.
Propane is a three carbon alkane and is commonly used for engines
and home heating systems. |