|
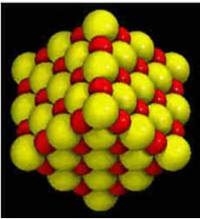
Sodium is half the size of
chlorine, creating a
“cubic pattern” |
CHEMICAL BONDING
|
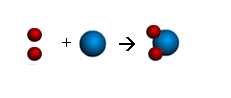
2 hydrogens (red) + 1 oxygen
(blue) yields H20 a water molecule

E. Frankland
|
Atoms of different elements combine to
form molecules of a particular substance. These molecules are held
together by forces called "chemical bonds." There are various
types of bonds and the way these bonds are arranged is unique to that
compound. The two major types of bonds between atoms are called
ionic (transfer of electrons) and covalent (sharing of
electrons). Some of the other forces that allow molecules to “bond” are
called Van der Waals Forces.
A compound consists of two or
more different types of atoms that are chemically bonded. Electrons
move around the nucleus of an element in specific and set orbitals.
There are a finite number of electrons in each of these orbitals.
For example, halite (NaCl or
table salt) is a combination of sodium and chlorine.
Halite crystals grow in a cubic form, which reflects how chlorine and
sodium combine. Since a sodium atom is half the size of an atom of
chlorine, the two elements form a perfect cubic pattern (see figure).
The sodium and chlorine share electrons making it an ionic substance.
Ionic compounds are weaker than covalent bonds. That is why when you
combine salt in water (H2O, a covalent bond), the salt
dissolved into its ionic state. Scientists were aware that familiar
substances changed their characteristics when performing experiments,
but they did not understand until the 1800’s how elements bond and how
they break and come back together. |