|
FAMILY OF MINERALS
The chemical composition of a mineral determines the
overall geometric arrangements of that mineral. How the atoms bond
with each other determines other properties including crystal shape,
cleavage, behavior of light, and hardness.
Chemical families are divided into classes
depending on the anion or positive radical that is part of their
chemical formula. A positive radical is a combination of elements that
have a positive charge and is a prominent part of the chemical formula
that makes up the mineral. You can think of a "family" with
the same last name. For example feldspar has a radical of Si3O8
and mica has a radical of Si3O10. Both have
silicon and oxygen in their family name, so they both belong to the
silicate family.
Chemical families help to group minerals to look
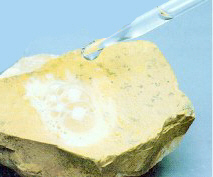
for similar properties. For example, "carbonatesí include the
radical "CO3 " which reacts with acids to produce
carbon dioxide. Minerals like calcite and azurite will produce a
"fizzing" as carbon dioxide bubbles are released when acid
like HCl is dropped on the mineral. Many oxides will react with the
atmosphere and will "rust." Limonite belongs to the oxide
family and has a characteristic rust color.
We will look at 6 positive radials in our chemical
family chart including oxides, sulfides, sulfates, carbonates,
silicates, borates, and halides. There are a few other groups of
chemical families that we have not included in this chart because they
are not common minerals.
|

Feldspar - a silicate

Mica - a silicate
|