BACKGROUND:
Salts originate from the erosion of
the land. Evaporation prevents salts from going into the clouds, so the
waters become full of various salts. The salt then precipitates if
there is more salt than the water can “hold” within the molecules of water.
You can explain this as referring to a large structure made of paper cups,
if there are too many cups, the weight of the cups will make all the cups
fall down. Precipitation is when the salt is “supersaturated” and
the remaining salt falls to the bottom or precipitates.
Salt water is salt mixed with fresh water.
However, salt in cold water does not dissolve as well as if the water is
warm. Warm water has more room between the water molecules, allowing
more salt to fit. Cool water molecules are tighter together
and will not allow much salt to dissolve.
PROCEDURE:
- Students will make salt from
water. In this lab ordinary table salt will be used, but in reality
the "salt" in salt water consists of various other compounds as will be
discussed in the post lab.
- Water can just take so much salt.
Have the students measure about 10 ml of water into a jar. Have the
students stir in 1 ml of salt. Discuss what happens. Provide
the students with warm water. Have them repeat the procedure with
the warm water. Discuss that the salt will dissolve more readily
in the warm water.
- Ask students if it is easy to tell
if a liquid has salt in it without tasting it. You may want to pour
some water from one source into a dish but do not label it. See if
the children can guess, from its appearance whether the water is salty
or fresh. Have them tell you why they guessed the way they did.
Put the water in a place where it can evaporate and have the students see
for themselves that the salt will be left behind.
- Students should measure about 100
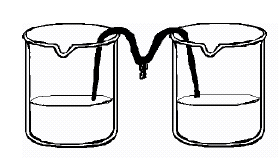
ml of warm water (with beaker) and mix with 5 ml of Epsom salt (with spoon)
into the water. They will pour half of the solution in another
jar and place one end of the cotton string (mop string) in each of the
two jars with Epsom salt solution, as shown in the diagram on the right.
Let the string dip between the jars and let the jars sit for several days.
The solution should flow from each jar toward the middle, where it will
drip, forming “salt” pillars. This will take a few days, so put the
beakers in a place where they will not be disturbed.