BACKGROUND:
Minerals are pure substances composed of one or more
elements. Internally, a mineral has a repeating atomic structure or
crystalline pattern. This structure is similar to the mathematical term
"tessellation" which refers to polygons that repeat themselves in
a pattern. The visible crystal shape of a mineral is due to this repeating
atomic pattern. If space is available, a mineral will grow in its
characteristic crystalline shape. If a growing crystal is constrained, it
will take on the shape of the space.
Some minerals break in a characteristic pattern,
called "cleavage," which is also caused by alignments in the
mineral’s atomic structure. Gemologists sometimes use cleavage patterns to
cut gems into faceted shapes.
It is sometimes difficult to tell whether a specimen
is a crystal or a broken mineral. This lab will allow students to touch and
see different mineral shapes. It is important that they begin to identify
the shapes, not whether they can identify whether it is a crystal or cleaved
specimen.
PROCEDURE:
- In the module you have ten specimens including quartz, amethyst,
pyrite, calcite, halite, fluorite, feldspar, mica, gypsum, and citrine.
You may want to include your own specimens.
- Review the shapes of a cube, dipyramid, tabular (3 dimensional
rectangle), rhombohedron, and six-sided prism. Use the pictures below to
help students identify different shapes. In the Post Lab, students
will look at more shapes. Draw a rhomohedron, cube, dipyramid,
six-sided pyramid (like a pencil top), and tabular (3-d rectangular,
book-shaped). Have the students draw the shapes on their worksheets. Explain
that some of the
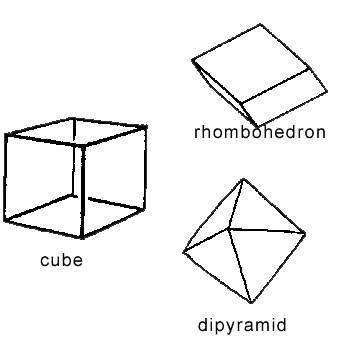 specimens are complete crystals, which may match the
shapes they have drawn, while others may only show fragments of the
shapes. Do not worry about whether they are cleaved or
crystals. specimens are complete crystals, which may match the
shapes they have drawn, while others may only show fragments of the
shapes. Do not worry about whether they are cleaved or
crystals.
- When the students are done, discuss the characteristics of the each
of the specimens, as listed below. Be sure to go over the shapes.
QUARTZ - found in quartz watches, one of the most common
minerals, crystals form 6 sided prisms, when it breaks it produces a
conchoidal or rounded break similar to broken glass
AMETHYST - purple-colored quartz, six-sided crystal shape
CITRINE - yellow- to brown-colored quartz, six-sided crystal shape
FLUORITE - a source of fluorine, dipyramid (octahedron) or cubic
(depending on specimen), specimen in module is cleaved
PYRITE - often called fools’ gold, used to make sulfuric acid,
cubic crystal shape
HALITE - common table salt, cubic crystal shape
CALCITE - commonly called iceland spar, can see double image,
rhombohedral cleaved surface in module, crystal shape resembles a
"dog tooth"
FELDSPAR - used in making some ceramics, rhombohedral cleaved
specimen
MICA - crystals are tabular or prismatic, but these specimens
show "sheet" cleavage
GYPSUM - rosette or tabular crystal shapes, they also cleave in
tabular pieces; this specimen is a crystal
|