|
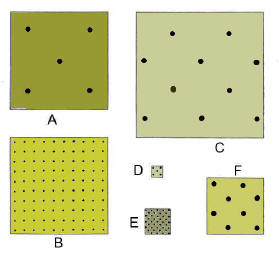
E is the most dense while
A is the least dense |
Density is a helpful property. For example
galena, a mineral of lead sulfide (PbS) is very dense. A geologist learns to
“heft’ a stone to “feel” the density. Precise measurement of the density of a
pure substance will aid in the identification of the substance. Density
equals the mass of an object divided by the volume of that object, measured in
grams per cubic centimeters (D=M/V).
The masses of atoms and the spacing between
the atoms, determine the density of a material. We think of density as the
lightness or heaviness of materials. It is a measure of the "compactness" of
matter. The density of lead is greater than that of ice, and that of ice is
less than water. Because the density of ice is less than water, ice floats.
Gold is very dense. Panning for gold uses this property to gold so the flakes
of gold settle out of the water. |