|
LESSON 4. Ions, Molecules, and
Compound (Lab)
|
Objective:
Students construct models of molecules. |
Materials:
Polylab Molecules
|
Teacher Notes:
In this exercise the students will make
models of 14 ions, molecules, or compounds. It is important to understand
the difference amongst these forms of matter so later they can build on
these concepts. The reader provides an understanding of the term
“model.”
|
ANSWERS:
-
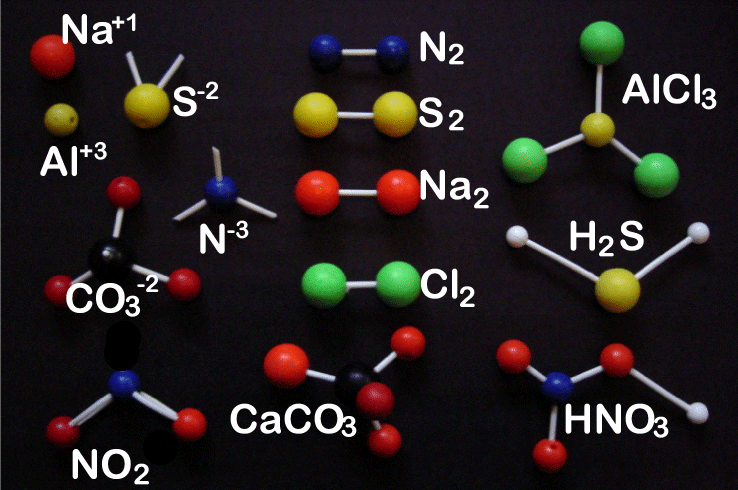
-
|
Formula |
Name |
type |
|
S-2 |
Sulfur ion |
Monatomic ion |
|
Al+3 |
Aluminum ion |
Monatomic ion |
|
Na+1 |
Sodium ion |
Monatomic ion |
|
N-3 |
Nitrogen ion |
Monatomic ion |
|
CO3-2 |
Carbonate ion |
Polyatomic ion |
|
Cl2 |
Chorine molecule |
Diatomic molecule |
|
Na2 |
Sodium molecule |
Diatomic molecule |
|
N2 |
Nitrogen molecule |
Diatomic molecule |
|
S2 |
Sulfur molecule |
Diatomic molecule |
|
NO2 |
Nitrogen dioxide |
Polyatomic compound |
|
AlCl3 |
Aluminum chloride |
Polyatomic compound |
|
HNO3 |
Hydrogen nitrate (nitric acid) |
Polyatomic compound |
|
CaCO3 |
Calcium carbonate |
Polyatomic compound |
|
H2 S |
Hydrogen sulfide |
Polyatomic compound |
|