|
Glenn Seaborg
an American chemist co-discovered plutonium in 1940. This lead
the way for a new group of radioactive elements called transuranium
elements (94-102). Seaborg placed the actinide series below the
lanthanide series which gave the Periodic Table the basic configuration
it has today. Seaborg and his laboratory developed techniques to create
synthetic elements. They also discovered 100 isotopes of other
elements. This added the last major change in the Periodic Table of the
Elements.
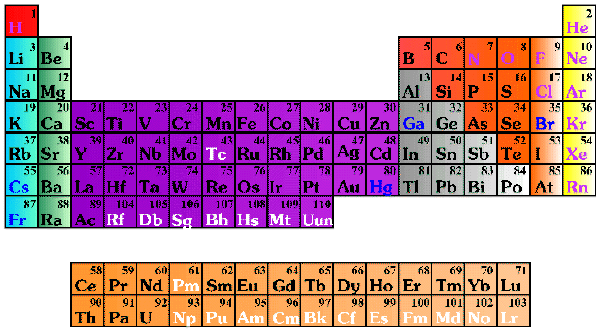
Today's basic "look" of the periodic table
The Periodic Table of the Elements is
a great example of how science has worked in the past and is continuing
to work today. Many scientists communicate discoveries to each other so
they can test and add more information. Science is an accumulative
subject that builds on the work of many people. Science is alive with
each new discovery which can change our understanding of a subject. |