
Sulfur ion (S2
) |
IONS,
MOLECULES, AND COMPOUND - LAB
PROBLEM:
Can models help understand ions, molecules, and compounds?
HYPOTHESIS:
MATERIALS:
Polylab molecule set

Chlorine diatomic molecule (Cl2)
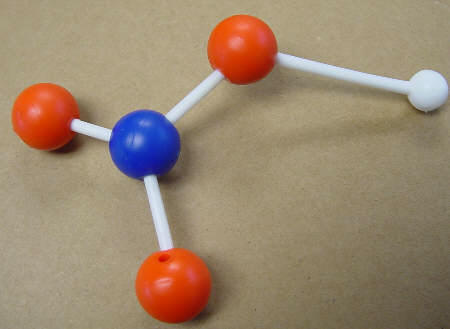
Hydrogen nitrate, a compound |
For this
exercise you will need the following color balls and connections:
Small orange
and red = oxygen (11); large green = chlorine (5) ; small yellow = aluminum (2);
large orange = sodium (4); small blue = nitrogen (5); large black = carbon
(2); small white connected with rod = hydrogen (3); large yellow = sulfur (4);
connections (27 ) *note: hole means it needs electrons (i.e. N-3 );
connector means it gives electrons (i.e. Al+3)
Build the
following molecular models looking at the formula on the table. Ions would have
an incomplete connection (see sulfur ion), diatomic molecules will have 2 of the
same connected, and after you have built them have your instructor check if
they are correct. For each of the molecules describe how the molecule is
different from the rest, how many atoms make up the molecule, and which elements
are parts of the molecule in the "conclusions."
|
Formula |
Name |
type |
Checked by instructor |
|
S-2 |
|
|
|
|
Al+3 |
|
|
|
|
Na+1 |
|
|
|
|
N-3 |
|
|
|
|
CO3-2 |
|
|
|
|
Cl2 |
|
|
|
|
Na2 |
|
|
|
|
N2 |
|
|
|
|
S2 |
|
|
|
|
NO2 |
|
|
|
|
AlCl3 |
|
|
|
|
HNO3 |
|
|
|
|
CaCO3 |
|
|
|
|
H2 S |
|
|
|
Draw the model for each of the ion, molecule, or compound that you created?
|
S-2
|
Al+3 |
Na+1 |
|
N-3
|
CO3-2 |
NO2-1 |
|
Cl2
|
Na2 |
N2 |
|
AlCl3
|
HNO3 |
CaCO3 |
|
H2 S |
NO2
|
|
|