|
IONS,
MOLECULES, AND COMPOUNDS
Procedure: use
the
Periodic Table Placemat
on some of the questions below and answer
the following questions.
-
Minerals are usually made of compounds. In the area below list the
cation and anion of the minerals listed with their chemical
formula. (The subscript number has nothing to do with figuring out
the cation and anion.)
|
MINERAL |
Chemical formula |
Cation |
Anion |
|
Hematite |
Fe2O3 |
Fe |
O |
|
halite |
NaCl |
|
|
|
quartz |
SiO2 |
|
|
|
pyrite |
FeS2 |
|
|
|
galena |
PbS |
|
|
|
fluorite |
CaF2 |
|
|
-
Why are
elements in Group 18 usually not part of a compound?
-
State
whether the following is a monatomic ion and polyatomic ion.
| |
Monatomic or Polyatomic? |
|
Fe+3 |
monatomic |
|
Fe+2 |
|
|
NO3-1 |
|
|
SO4-2 |
|
|
Na+1 |
|
|
PO4-3 |
|
|
Al+3 |
|
|
CO3-2 |
|
|
H+1 |
|
|
Sn+4 |
|
|
OH-1 |
|
-
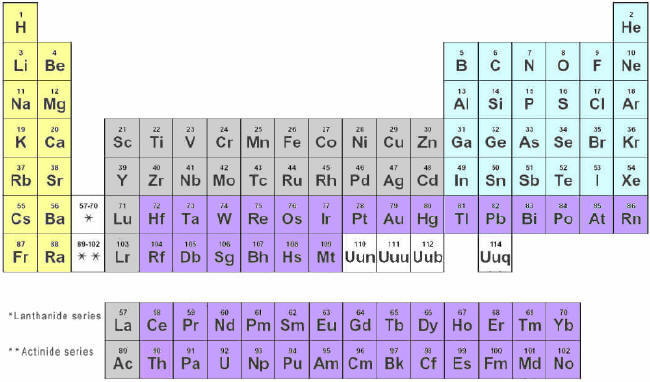
Look
at the recommended Periodic Table of the Elements find bolder lines
that divide the simplified “metals” with “nonmetals” that generalizes
which groups are the cations and which ones are usually anions, but
could have cations. On the table below show that division. Color
anions red and cations blue (only naturally occurring).
Note
that Noble Gases do not usually combine, so exclude that group.
-
Look at
the table below and determine if it is likely that these 2 elements
could be part of a compound.
|
Element 1 |
Write element |
Element 2 |
Write element |
Could this become a compound |
|
Fe |
Iron |
Co |
Cobalt |
no |
|
Ca |
|
O |
|
|
|
Re |
|
Ir |
|
|
|
Ce |
|
Ru |
|
|
|
Ca |
|
F |
|
|
|
Mg |
|
O |
|
|
|
Ba |
|
La |
|
|
|
Na |
|
Mg |
|
|
|
Na |
|
I |
|
|
|
W |
|
Xe |
|
|
|
Au |
|
Ag |
|
|
|