ELEMENTS - LAB
 |
|
Copper |
In our everyday world, we see and use
"elements" in many ways. Advertisements talk about, "Iron
is needed for your blood" or people refer to a "Silicon
Valley," but few people think of iron or silicon as elements.
Unfortunately, not all references to elements are scientifically
accurate. For instance, lead in a pencil is not the element lead,
but the mineral graphite which is composed of the element
carbon. A five-cent nickel in American currency only has a small
percentage of the metal nickel in it. The “calcium” they
refer to in advertisements (needed for strong bones in milk) is not the
element calcium, but a compound (mainly calcium phosphate). The
calcium ion (Ca2+) is what is needed for the bones to
incorporate into its structure. Elemental calcium is a silver white
metal and is very reactive with water.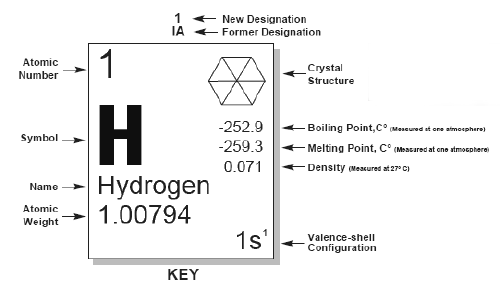
Elements have
unique physical and chemical properties. A physical
property would include characteristics like color or density,
a chemical property would include reactions at the atomic level like its
crystal structure or atomic structure. An atom only has
a composition made of one substance. A single atom is unstable and
usually forms a partnership or bonds with itself or other elements.
This is called a molecule, which is the stable form that allows
us to observe elements in nature. The individual boxes on the Periodic
Table of the Elements, helps describe some of the physical and chemical
properties of an individual element. If you look at the Hydrogen box (Key)above,
you can find the chemical properties such as Atomic Number,
Atomic Weight (or what we will refer to as Atomic Mass),
Crystal Structure, and Valence-shell configuration. Density,
Boiling Point, and Melting Point define some of its physical
properties. |