|
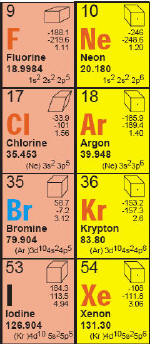 Many
Periodic Tables contain a valence-shell configuration. Valence
electrons are contained in the outermost shell or energy level, of
an atom. They are important because they can help predict how that an
element reacts chemically with other elements. The outer valence
shell can be predicted by the way the Periodic Table of the Elements
is constructed. In Lesson 2 you will learn more about these shells,
but for now we just want to read them on the table. If you look at
Iodine on the table is written as (Kr)4d105s25p5.
The Kr refers to the structure of Krypton, an element in
Period 4. If you look at Krypton’s valence-shell configuration it
contains and Ar (Argon) before the structure and if you look at
argon it has Ne (Neon) in its formula. The Neon does
not have He (Helium) written before it, but that is because there
is room to write helium’s structure of 1s2. So back to
Iodine, its entire formula would be written as: 1s22s22p63s23p63d104s2
4p64d105s2 5p5. That
is too much to put in one box. Many
Periodic Tables contain a valence-shell configuration. Valence
electrons are contained in the outermost shell or energy level, of
an atom. They are important because they can help predict how that an
element reacts chemically with other elements. The outer valence
shell can be predicted by the way the Periodic Table of the Elements
is constructed. In Lesson 2 you will learn more about these shells,
but for now we just want to read them on the table. If you look at
Iodine on the table is written as (Kr)4d105s25p5.
The Kr refers to the structure of Krypton, an element in
Period 4. If you look at Krypton’s valence-shell configuration it
contains and Ar (Argon) before the structure and if you look at
argon it has Ne (Neon) in its formula. The Neon does
not have He (Helium) written before it, but that is because there
is room to write helium’s structure of 1s2. So back to
Iodine, its entire formula would be written as: 1s22s22p63s23p63d104s2
4p64d105s2 5p5. That
is too much to put in one box.
Notice that there
is a pattern developing. There are “5” shells, and each shell has a
sequence of a s, p, and d. Complicated? Only if
you don’t see the pattern! Let’s look at the table closely. Group 1 (alkali
metals) and 2 (alkaline earth metals) contains its valence
shell in the “s” section. Group 3-12 (transitional metals)
contains the valence electrons is both the “s” and “d” section. There
are a few exceptions, but most of the transitional metals have two
electrons in the outer shell.
Groups
13-18 have their valence electrons in the “p” section. The Lanthanide
and Actinide Series all contain the “f” section. However
Lanthanum and Actinium do not.
|