|
CHEMICAL FORMULAS
Make
the following
models using the Polylab set show them to your instructor to make sure
they are correct.
- Methane (CH4)
- single bond – look at article for help
-
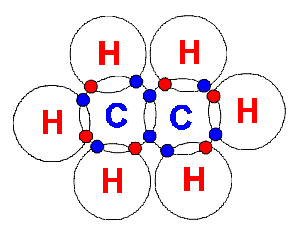 The
6 Hydrogen atoms and the 2 Carbon atoms combine to form Ethane (C2H6).
This is a single bond. The
6 Hydrogen atoms and the 2 Carbon atoms combine to form Ethane (C2H6).
This is a single bond.
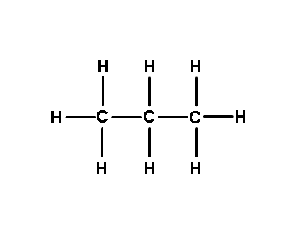
- Propane(C3H8)
This method can be continued to form Propane (C3H8),
which is an inflammable liquid. Campers often use Propane in lamps
and heaters. This is a single bond.
- Butane (C4H10)
is a highly flammable, colorless, odorless and easily liquefied. It is
single bonded (alkane). (No help on this one, try and figure out how
to
make.)
- Ethylene (double
bond) - C2H4 (look at reader)
- Acetylene (triple bond) - C2H2
(look at reader)
|