|
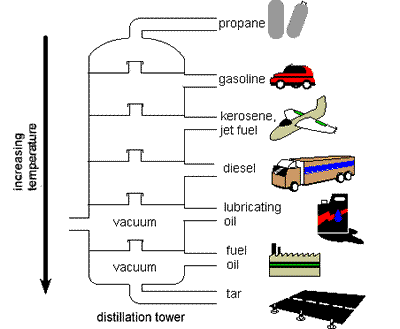
Products from
hydrocarbons |
Probably the most important
product of the fractional
distillation of
petroleum
is gasoline, a mixture of alkanes containing six to ten carbon atoms in
their molecules: hexane (C6H14),
heptane (C7H16),
octane (C8H18),
nonane (C9H20),
and decane (C10H22),
plus small amounts of higher-molecular
weight alkanes. More than six trillion gallons of gasoline are burned
each year in the United States.
Of all the hydrocarbons that
can be in gasoline, normal heptane, C7H16,
has been found to make
auto engines
knock worst. (in other words, not good for the engine). It has been
assigned a value of
zero on a scale of gasoline desirability. The hydrocarbon that
knocks least is a branched-chain form of octane, C8H18,
called iso-octane. It has been rated 100. Every gasoline blend is
assigned an octane rating between zero and 100, according to how
much knocking it produces under standard test conditions. Most
automobile fuels sold have octane ratings above 85.
High-octane
gasolines that are even better than iso-octane because of anti-knock
additives can have ratings above 100. So
even
buying gasoline has something to do with the chemical formula of alkane
hydrocarbons! Look at the octane the next time you go to a gasoline
station. |